Nutrition Information on FDA Labels: Promoting Health and Regulatory Compliance
June 28, 2023

Be among the first to receive our articles from our blog.
Introduction
Nutrition is a topic of vital importance in our daily lives. For this reason, the US Food and Drug Administration (FDA) places special emphasis on Nutrition Information on product labels sold in the country. In this article, we will explore the importance of this information, recent changes in labels, and how FastForward, an FDA registration agency and labeling review specialist, can help you efficiently and accurately comply with FDA requirements.
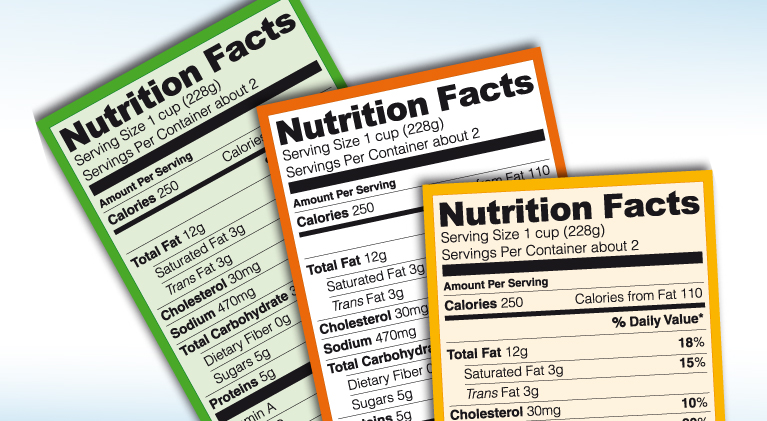
The new nutrition information label
Recently, the FDA launched an updated nutrition information label, which is now mandatory for food and drug manufacturing, packaging, and labeling companies. This update is based on the latest scientific findings to help consumers understand the relationship between diet and the onset of chronic diseases.
What should the nutrition information label contain?
The nutrition information label aims to provide consumers with the necessary data to make long-term healthy choices. Some important elements that should appear on the label are:
Serving size: Indicates the number of servings per package and the size of each serving. Calorie amount: Shows the total calories per serving and calories from fat. Daily value: Informs how the nutrients in a serving contribute to the total daily diet. Nutrient limits: Reveals the percentage of saturated fat, trans fat, and sodium per serving, as excess amounts can increase the risk of chronic diseases.
The role of the FDA in consumer education and guidance:
The FDA aims to guide consumers toward healthy eating. Through nutrition information on labels, key information about calories, serving sizes, added sugars, nutrients, and daily values is provided. This allows consumers to make informed decisions to prevent or control diseases such as type 2 diabetes, certain types of cancer, heart diseases, obesity, high blood pressure, and osteoporosis.
FastForward: Your ally in FDA compliance:
FastForward is an agency specializing in FDA registrations and labeling review. Our team is trained to work in both English and Spanish, and we offer efficient and agile services. By choosing us, you can benefit from:
Thorough review: We check various aspects to ensure compliance with FDA regulations, including information accuracy and clarity, presence of mandatory label elements, and more. Quick response time: We strive to provide you with results in a reduced time frame. The review process usually takes between 5 to 9 business days. Avoiding legal consequences: Complying with FDA labeling requirements is crucial to avoid legal issues and protect your company’s reputation. Our team will help ensure that your products meet all necessary regulations. Advice and guidance: If you have additional questions about product labeling according to the FDA, our team of experts is available to provide advice and additional guidance. You can contact us through our website or request a free consultation call.
Conclusion:
FDA Nutrition Information on Labels is essential for consumers to make informed decisions about their diet. The FDA cares about consumer health, and FastForward is committed to helping you comply with labeling regulations efficiently and accurately. Don’t risk your product or reputation! Contact us and discover how we can support you in FDA regulatory compliance.
FAQs:
Does the label need to be in English for the review?
Not necessarily. At FastForward, we can review labels in both English and Spanish. Our team is trained to work with both languages.
Can I copy a label from a product already sold in the United States?
No, copying a label from a product already sold in the United States does not guarantee FDA compliance. This can have serious consequences, such as detentions or product recalls from the market. It’s important to ensure full compliance with all FDA labeling regulations.
What is checked on the label?
In label reviews, we verify various aspects to ensure compliance with FDA regulations. This includes information accuracy and clarity, the presence of mandatory information such as ingredient lists, allergen declarations, nutrition information, warnings, usage instructions, among others.
How long does the review take?
The review time can vary depending on the quantity and complexity of the labels being reviewed. At FastForward, we strive to provide efficient and agile services. Once we receive the labels, our team will work diligently to complete the review in the shortest possible time. Usually, it takes between 5 to 9 business days.
What happens if my product doesn’t comply with FDA labeling requirements?
If your product doesn’t comply with FDA labeling requirements, it may face legal and regulatory consequences. These can include product retention or rejection at customs, fines, lawsuits, or even a ban on product marketing. It’s crucial to comply with regulations to avoid issues and protect your company’s reputation.
What is considered deceptive/misleading advertising in labeling according to the FDA?
The FDA considers deceptive/misleading advertising in labeling any false or misleading statement that can mislead consumers regarding the safety, quality, or effectiveness of a product. This includes scientifically unsupported claims, incorrect information about ingredients or product benefits, among other potential deceits. Complying with regulations and avoiding deceptive advertising is essential for regulatory compliance and consumer trust.
How can I get additional advice or guidance on product labeling according to the FDA?
At FastForward, we are here to provide you with the necessary advice and guidance regarding product labeling according to FDA regulations. You can contact us through our website or request a free consultation call. Our team will be happy to answer your questions and provide the guidance you need.
Escríbenos por Whatsapp

Si no renueva su registro antes de las 23:59 (EST) del 31 de diciembre, su número será eliminado de la base de datos de la FDA

¿Necesita ayuda?